Childhood Cancer R&D Program: Developing and Accelerating Novel Therapies for Children With Cancer
Sanofi is working to address childhood cancer as a global health issue and to improve survival rates for children with cancer. The Sanofi Corporate Social Responsibility (CSR) Childhood Cancer R&D program focuses on accelerating and developing novel therapies for children with cancer.
Children are our future, and it is our duty to protect them from illness. As a pharmaceutical industry, we take pride in fostering innovation for Novel and improved therapies for children with cancer. By leveraging our expertise and partnering with specialists, we synergize our efforts. Together, we can do more!

Iris Valtingojer
Childhood Cancer Research Lead - R&D
Childhood Cancer in Numbers
400,000
Over the past 60+years, survival rates for children with cancer have greatly improved, most notably for most leukemias and lymphomas, along with a number of solid tumors such as Wilms tumor, a tumor of the kidney. Despite this progress, a number of childhood cancers, subtypes of certain cancers, or cancers that relapse, often lack curative treatments [1]. Furthermore, the majority of children who survive cancer, experience one or more significant long-term side effects later in life as a result of treatments that may include intensive chemotherapy, radiotherapy or surgery [2] [3].
Childhood cancers, which comprise only 1% of all cancers, exhibit distinct biologic traits that usually present rapidly and follow an aggressive growth pattern. Under the microscope, many childhood cancers contain features seen in fetal tissues and are the result of dysregulated development [4]. Compared to adult cancers, childhood cancers harbor fewer genetic mutations but generally show more gene fusions and epigenetic changes [5].
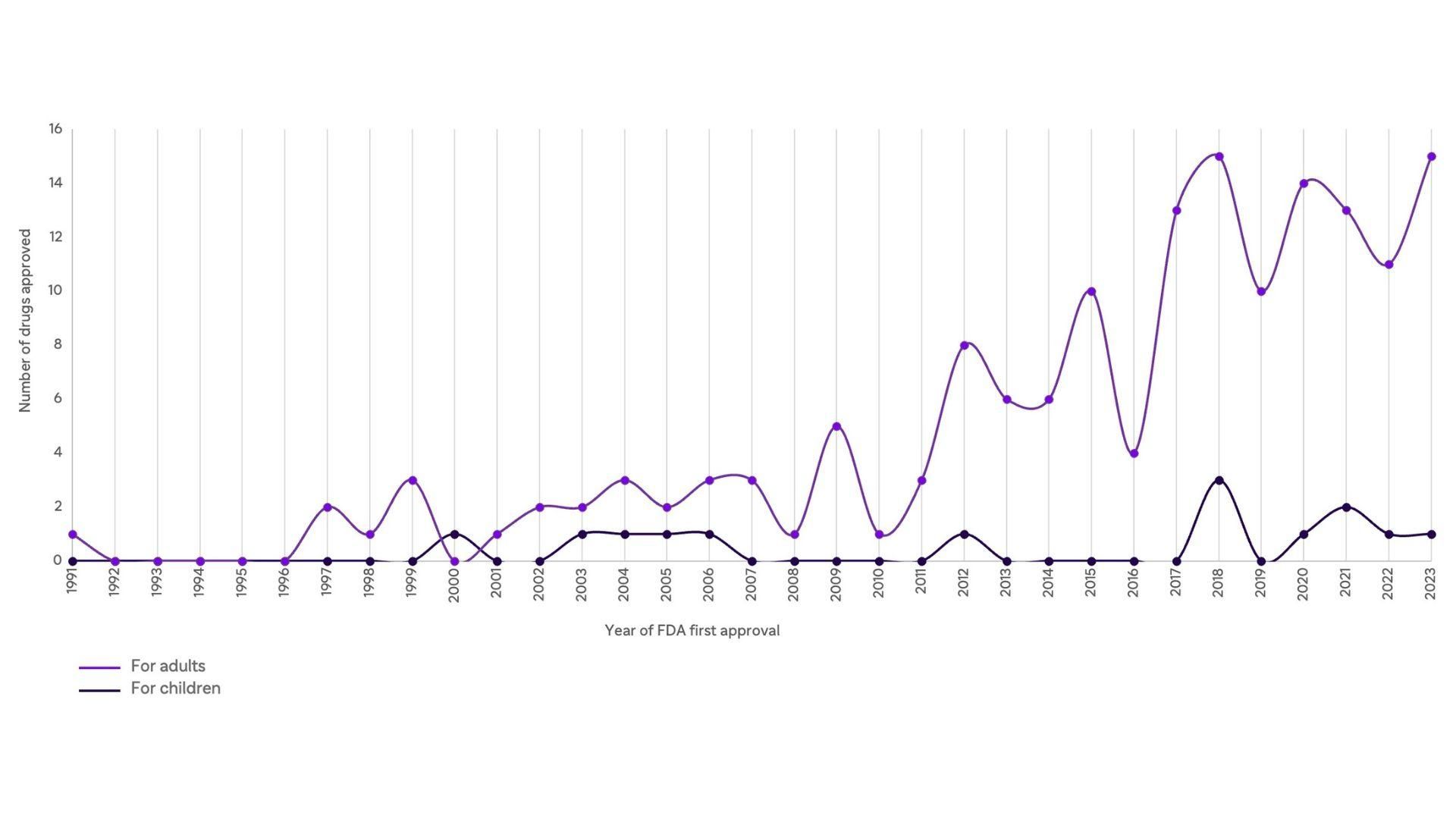
Developing new treatments for childhood cancers has been challenging as each cancer is rare or ultra-rare, lacking clear economic models that can support research & development in the private sector. Despite legislation in the US and Europe that incentivize or require the conduct of pediatric studies [6], clinical trials for children still encounter a 6.5-year delay compared to adults [7].
Targeted Cancer Therapies Approved by the FDA Over Time
Childhood Cancer R&D Program: Our Aims
The goal of this program is to promote the development of novel and safer drugs for children with cancer, to accelerate the start of clinical trials for children whenever scientifically appropriate, and to become a role model for other pharma companies by showing that the pharmaceutical industry can help lead development of new treatments under the umbrella of corporate social responsibility.
Childhood Cancer R&D Program: How We Do It
Leveraging Sanofi’s R&D Pipeline
We implemented a proactive approach to identify drugs from the Sanofi R&D pipeline that could be of potential benefit for children with cancer. By systematically evaluating the pipeline early in the R&D value chain, we identify compounds targeting pathways that are relevant for childhood cancer. We conduct preclinical studies and initiate protocols for clinical trials with children once the scientific relevance is confirmed. In 2023, we thus started a clinical trial in children with leukemia in less than 2 years after adults, an impressive improvement compared to the median of 6.5 years delay between trial initiation in children versus adults.
Generating Knowledge Through Partnerships and Collaborations
We want to accelerate drug development for children with cancer by joining forces and sharing knowledge with expert institutions and consortia in the field. To this end, we have built strategic partnerships and collaborations with a number of premier organizations, including but not limited to the Innovative Therapies for Children with Cancer (ITCC) and the Foundation for the National Institutes of Health (FNIH) COACH consortia. In 2023, together with ITCC, we have launched a multistakeholder Childhood Cancer Working Group at the Paris-Saclay Cancer Cluster (PSCC). One of the aims of this PSCC Childhood Cancer Working Group is to encourage biotech- and start-up firms to work on childhood cancer.
In 2024, we co-organized the first-ever hackathon dedicated to childhood cancer, Building on the momentum of our collaborative efforts. Ten promising projects were pre-selected to participate, with around 30 experts from various fields providing mentorship, guidance, and feedback to help refine their solutions.
Highlights and Partners

We also foster innovation in childhood cancer research through Sanofi's i-Awards / iDEA-iTECH Awards and the Sanofi Golden Ticket Initiative.
Additionally, in collaboration with Life Arc, we authored a book chapter on collaborative innovations in childhood cancer therapies, emphasizing how collective action can accelerate therapeutic breakthroughs.8
Raising Awareness with Symposia and Events
Raising awareness about childhood cancers and the urgent need for new therapies is a top priority for us. We therefore organize events at Sanofi throughout the year, including specialized webinars on different research topics and informative seminars focused on childhood cancer. Additionally, every September during childhood cancer awareness month, we host an annual symposium where we gather key opinion leaders in the field. In 2024, for the first time, the event was open to external guest fostering broader discussion.
We also actively participate in international conferences and use these platforms to inform on our dedication to childhood cancer and to exchange with the community in the field. These events are invaluable opportunities for collaboration and knowledge exchange.
References
- Adamson, P.C., Improving the outcome for children with cancer: Development of targeted new agents. CA Cancer J Clin, 2015. 65(3): p. 212-20.
- Norsker, F.N., et al., Late Effects in Childhood Cancer Survivors: Early Studies, Survivor Cohorts, and Significant Contributions to the Field of Late Effects. Pediatr Clin North Am, 2020. 67(6): p. 1033-1049.
- Chang, W.H., et al., Late effects of cancer in children, teenagers and young adults: Population-based study on the burden of 183 conditions, in-patient and critical care admissions and years of life lost. Lancet Reg Health Eur, 2022. 12: p. 100248.
- Filbin, M. and M. Monje, Developmental origins and emerging therapeutic opportunities for childhood cancer. Nat Med, 2019. 25(3): p. 367-376.
- Grobner, S.N., et al., The landscape of genomic alterations across childhood cancers. Nature, 2018. 555(7696): p. 321-327.
- Vassal, G., T. de Rojas, and A.D.J. Pearson, Impact of the EU Paediatric Medicine Regulation on new anti-cancer medicines for the treatment of children and adolescents. Lancet Child Adolesc Health, 2023. 7(3): p. 214-222.
- Neel, D.V., D.S. Shulman, and S.G. DuBois, Timing of first-in-child trials of FDA-approved oncology drugs. Eur J Cancer, 2019. 112: p. 49-56.
- Valtingojer, I. et al., Collaborative Innovations in Childhood Cancer Therapies. In: Michel, M.C., Wieland, H.A. (eds) Public-Private-Partnerships in Drug Research and Development. Handbook of Experimental Pharmacology, 2024. vol 286. Springer, Cham. https://doi.org/10.1007/164_2024_725




