THIS SITE IS FOR HEALTHCARE PROFESSIONALS ONLY
This material is for scientific purposes only and is intended only for healthcare practitioners. Patient enrollment is dependent on approval by local ethics committees. The agent, amlitelimab, mentioned here is investigational and has not been approved by the US Food and Drug Administration (FDA) or any other regulatory agency worldwide for the uses under investigation. No conclusions regarding safety and efficacy should be drawn for such agents.

Welcome
This site provides information on OCEANA - the Phase 2 and 3 clinical development program in moderate-to-severe atopic dermatitis (AD) for amlitelimab, a fully human, non-depleting anti-OX40-ligand monoclonal antibody.
Studies in patients with moderate-to-severe AD
Amlitelimab is currently being investigated in patients with moderate to severe atopic dermatitis.

Stream-AD
Phase 2b Trial

Hydro
Phase 2 trial evaluating the effect of amlitelimab on vaccine antibody responses

Atlantis
Phase 2 trial evaluating long-term safety, tolerability, and efficacy of amlitelimab

Coast 1
Phase 3 trial in AD as monotherapy

Coast 2
Phase 3 Trial in AD as monotherapy

Aqua
Phase 3 trial in AD for patients with inadequate response to biologic or oral JAK inhibitor therapy

Shore
Phase 3 Trial in AD for patients on background topical corticosteroids

Estuary
COMING SOON

River-AD
Phase 2 long-term extension trial
Are you interested in becoming an Investigator for Sanofi Clinical Trials? Let us know
What is Atopic Dermatitis?
Atopic dermatitis (AD) is a heterogenous, common, chronic inflammatory skin disease and presentation varies by age, severity, and ethnic background.
Why are we doing these trials?
This program aims to evaluate the efficacy and safety of a potential therapy, amlitelimab, in patients with moderate to severe atopic dermatitis.
What is the OX40 Ligand Pathway and why is it important in AD?
Signs and symptoms of AD are the result of marked T-cell mediated local and systemic inflammation. Activated T-cells (e.g. Th2, Th17, Th22) are a key source of inflammatory cytokines including IL-4, IL-13, IL-17, IL-22 and IL-31. 1-3 OX40L-OX40 signaling plays a role in the chronicity of inflammation in AD by promoting effector T-cell survival, proliferation, and cytokine release.10
AD Inflammation originates with antigen presenting cells (APCs) which activate and instruct T cells via a series of upstream signals.2,4-6 Following activation, secondary co-stimulatory molecules, OX40L (within 24 hours) and OX40 (1-5 days), become expressed on APCs and T cells, respectively.7 The OX40L-OX40 signaling pathway promotes T cell proliferation, survival and cytokine release in AD, amplifying and sustaining T-cell mediated inflammation (type 2 and non-type 2).2,5
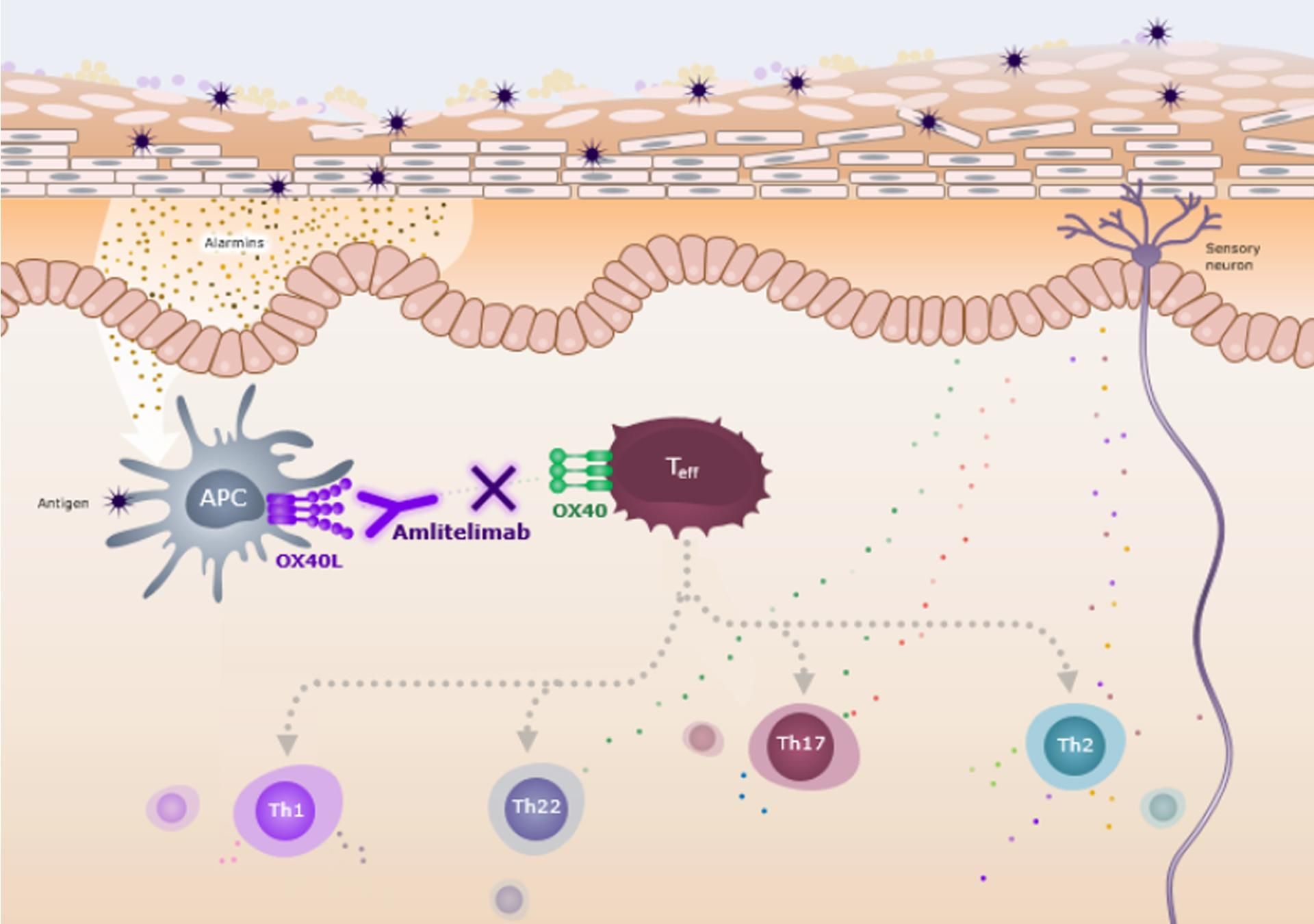
APC, antigen-presenting cell; OX40L, OX40 ligand; Teff, effector T cell; Th, helper T cell.
There are other cell types that express OX40L; however, the impact of amlitelimab on these interactions is not yet understood. The clinical significance of this mechanism of action is under investigation.
What is amlitelimab?
Amlitelimab is a fully human, non-depleting, anti-OX40L monoclonal antibody that binds to OX40L on APCs, preventing interaction with OX40 on activated T cells.8,9
Play video to learn more about amlitelimab's mechanism of action.
The agent, amlitelimab, mentioned here is investigational and has not been approved by the US Food and Drug Administration (FDA) or any other regulatory agency worldwide for the uses under investigation.
No conclusions regarding safety and efficacy should be drawn for such agents. There are other cell types that express OX40L; however, the impact of amlitelimab on these interactions is not yet understood. The clinical significance of this mechanism of action is under investigation.
FAQs
How can I learn more about the OCEANA Clinical Trial Program?
To learn more, please submit a request from medical information or request to connect with your local MSL: https://www.sanofimedicalinformation.com/
How can I partner with Sanofi beyond clinical trials?
If you are interested in submitting grants for research or are interested in collaborating with Sanofi, please visit our website for the ORIGINS program.
Are there other clinical trial programs for amlitelimab?
Amlitelimab is currently being investigated for hidradenitis suppurativa (phase 2) and asthma (phase 2b). Amlitelimab is also an active treatment arm in the CONQUEST study, a platform clinical study to evaluate investigational products for Interstitial Lung Disease Secondary to systemic sclerosis. This study is sponsored by the Scleroderma Research Foundation. Learn more.
As a healthcare provider, where can I find more information on Sanofi studies for my patients?
If you are a resident of the United States, the United Kingdom, or Australia seeking more information on Sanofi's ongoing clinical trials, please visit SanofiStudies.com for more information.
References
- Langan S, et al. Lancet. 2020;396:345–360;
- Furue M, et al. J Clin Med. 2021;10:2578–2586;
- Krohn IK, et al., Allergy. 2021;77(3):827-842
- Fu Y, et al. Acta Pharm Sin B. 2020;10(3):414–433;
- Goronzy JJ, et al. Arthritis Res Ther. 2008;10 Suppl 1:S3;
- Tai Y, et al. Front Pharmacol. 2018;9:642
- Croft M, et al. Immunol Rev. 2009;229:173−191
- Tkachev V, et al. Sci Transl Med. 2017;9:eaan3085
- Saghari M, et al. Clin Pharmacol Ther. 2022; 111(5):1121−1132
- Weidinger S, et al. Br J Dermatol. 2023;189(5):531-539